Isolator Chamber for Sterility Tests
The development of an isolator chamber dedicated to sterility testing aims to optimise sterilisation and bio-decontamination processes in a controlled environment. This specific chamber enables key process parameters to be analysed and improved, guaranteeing effective, reproducible decontamination.
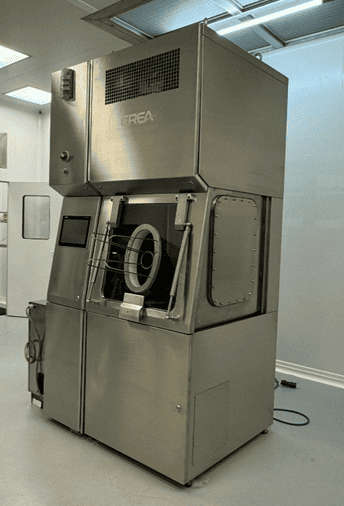
Technology and functions
Benefits and applications
Documentation
Technology and functions
- Totally isolated environment for reliable, reproducible tests.
- Optimisation of bio-decontamination parameters, including :
- Injection time and quantity of sterilising agents.
- Homogeneous distribution of the decontamination product.
- Control of environmental conditions for optimum efficiency.
- Integrated sensors for precise monitoring of critical parameters.
- Flexibility and adaptability, enabling different sterilisation protocols to be evaluated according to specific needs.
Benefits and applications
The use of an isolator chamber for sterility testing allows complete control of the bio-decontamination process, reducing the risk of residual contamination and improving compliance with the most stringent pharmaceutical standards.
This technology is an essential asset for the development and validation of sterilisation processes, guaranteeing maximum safety in aseptic environments.
Want to find out more about EREA innovations?
Our R&D team plays an essential role in the innovation and development of our industrial solutions.