Annex 1 GMP 2023 – Impact of its Update for 2024
Annex 1 of the Good Manufacturing Practices (GMP) sets out the rules for the manufacture of sterile medicinal products. The 2022 version finally comes into force in August 2023, after the 2008 version was the subject of numerous comments and revisions.
The revised Annex 1 is more demanding in order to ensure product quality and better protect patients. Is your company already compliant with Annex 1 GMP 2023? Call on EREA to help you.

Customised Technical Solution

Static and Dynamic Protection

Rapid Installation and Implementation

Easy-to-clean Surface
Would you like an estimate for your project?
Don't hesitate to contact us for a personalised quote.
They Trust Us
Main Changes to Annex 1 GMP 2023
Annex 1 GMP 2023 applies to the manufacture of sterile medical products. It contains 59 pages. The various stakeholders requested an in-depth revision of the version published in 2008 in order to adapt the regulations to ongoing changes in manufacturing. Annex 1 has been updated to improve product quality and to minimise the frequency and severity of product recalls and protect patients.
Many changes have been made by the updated GMP Annex 1. These include new requirements for Quality Risk Management (QRM) and Contamination Control Strategy (CCS). The new regulations emphasise the importance of these two points in manufacturing processes in controlled environments.
In addition, Annex 1 of the GMP takes into account new technological advances designed to optimise the manufacturing process for sterile products. It admits the use of new technologies, such as isolators and restricted access barrier systems (RABS), to modernise the regulations and the manufacturing process. Finally, the new update imposes new requirements in terms of disinfection, monitoring, evolution, cleaning and training.
The changes introduced by the updated GMP Annex 1 will come into force in August 2023. Stakeholders, including the pharmaceutical industry, must take steps to comply with these new requirements.
Highlights of the New Directives
Annex 1 GMP 2023 applies to sterile products. They cover a number of key points:
- The inclusion of Quality Risk Management (QRM), being a systematic process for assessing, communicating, controlling, monitoring and controlling the quality risks of pharmaceutical products throughout their life cycle.
- The inclusion of new technologies and innovative processes to guarantee the sterility of medicines. Pharmaceutical companies can do without the usual cleanrooms and equip themselves with more efficient and cost-effective equipment.
- Implementing a contamination control strategy (CCS): manufacturers of pharmaceutical products are required to implement a contamination control strategy. Contamination control must comply with regulatory requirements. It covers cleaning, disinfection and decontamination.
Annex 1 GMP 2023: challenges solved by EREA Pharma
Call on EREA to help you meet the challenges associated with your pharmaceutical production activities governed by Annex 1 GMP 2023. We offer tailor-made support to help you prepare before the new directives come into force. Our team of experts can identify your needs and provide you with the equipment you require to ensure that your drug manufacturing process complies with Annex 1 GMP 2023.
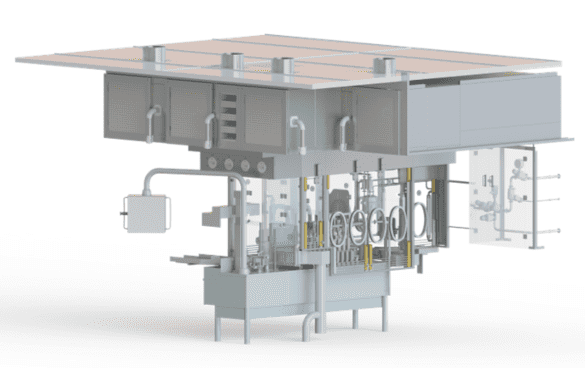
We design and manufacture a range of solutions to protect your sterile processes from contamination, including isolators and restricted access barrier systems. Depending on your requirements, we can supply customised equipment.
Find out more about our services and products by contacting our technical experts!
Need a specific configuration? Customised dimensions?
Discover our insulators, tailor-made to meet your requirements.















