Distribution isolator
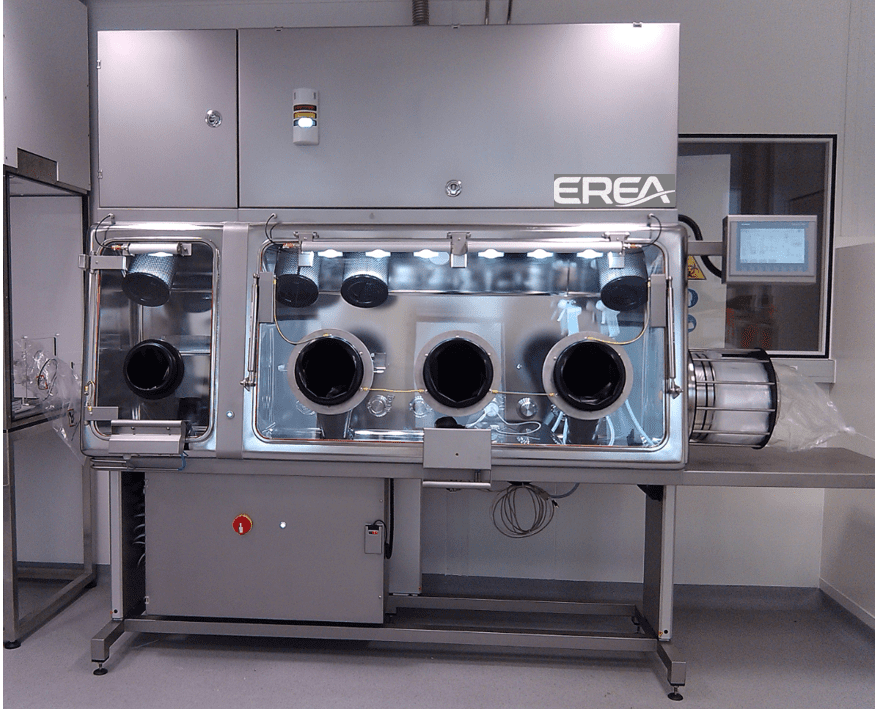
The handling and manufacture of radiopharmaceuticals is not without risk. As such, it requires extreme rigour to ensure the safety of operators and researchers, as well as product quality. The distribution isolator, which complies with Good Manufacturing Practices (GMP), is the ideal tool to meet these requirements. Are you involved in the radiopharmaceutical industry? Then you can’t do without this renowned tool. Discover its features here.

Customised Technical Solution

Static and Dynamic Protection

Rapid Installation and Implementation

Easy-to-clean Surface
Would you like an estimate for your project?
Don't hesitate to contact us for a personalised quote.
They Trust Us
Features and Benefits of Distribution Isolators
The distribution isolator for radiopharmaceutical products incorporates advanced features to ensure the safe and efficient handling of these products. This type of tool is primarily equipped with sophisticated air filtration systems. The distribution isolator therefore provides maximum protection against contamination. The gloves installed inside the isolator also allow for precise handling of various products. As a result, exposure risks are reduced.
In addition, isolators are in most cases designed with materials compatible with radiopharmaceutical products, minimising unwanted interactions. Among the advantages of this tool is its ability to offer maximum protection to operators, particularly against radiation. There is also a significant reduction in the risk of cross-contamination. Finally, the quality of the radiopharmaceutical products distributed is optimally preserved. It should be noted that isolators offer great operational flexibility. This facilitates rapid changeover between different distribution tasks.
Maintenance and Regulatory Compliance of Distribution Isolators
As with any other professional tool, the maintenance of a distribution isolator for radiopharmaceutical products must not be neglected. This ensures its continued performance and the safety of various operations.
A regular maintenance schedule, including performance tests and checks of the filtration systems, is essential. Maintenance of gloves and internal surfaces in particular must be carried out with special care to prevent contamination. Regulatory compliance is another critical aspect.
More specifically, isolators must comply with the GMP standards set by regulatory authorities. Comprehensive documentation of maintenance procedures, periodic validations and quality controls is required to demonstrate compliance. Regular internal and external audits are also recommended to ensure compliance with current standards.
Handling and Safety Challenges in the Distribution of Radiopharmaceuticals
The handling and distribution of radiopharmaceuticals present specific safety challenges due to the radioactive nature of the substances used in nuclear medicine. These products are essential for the diagnosis and treatment of certain diseases. However, they require rigorous precautions to protect staff, patients and the environment from radiation exposure. The safety of the various operators is a priority, which requires the use of specialised isolators and protective equipment that ensure handling in a sterile, secure and completely isolated environment.
Radiopharmaceutical distribution isolators are also designed to meet safety and contaminant control standards. They create a physical barrier between the operator and the product, allowing radioactive substances to be handled without direct exposure. These devices must therefore be equipped with highly efficient filtration systems and radiation-resistant materials to ensure a safe and clean working environment. It should be noted that the management of radioactive waste and any emissions is also an important issue, requiring appropriate containment solutions.
In addition to safety, the requirement for precision in distribution is also one of the challenges involved. More specifically, the preparation and packaging of radiopharmaceutical products must be carried out under perfectly sterile conditions to avoid any cross-contamination. Each stage of handling must be carefully controlled to ensure accurate and reliable administration of doses to patients.
Modern isolators incorporate cutting-edge technologies to meet these challenges, offering tailor-made solutions adapted to the needs of each laboratory. Process safety and product quality are therefore guaranteed.
EREA: Innovation in Distribution Isolators
Innovation in distribution isolators is embodied by EREA technology. Our isolators incorporate advanced radiation detection systems, enabling real-time monitoring of radiation exposure. This technology provides a double layer of safety, both for the various players in the pharmaceutical industry and for the products being handled.
Our various products also feature humidity and temperature sensors, ensuring an optimal environment for radiopharmaceutical products. In addition, the use of innovative materials significantly reduces the weight of each isolator, greatly improving manoeuvrability while maintaining structural strength.
In short, the GMP-compliant distribution isolator for radiopharmaceutical products is essential for ensuring the safety, quality and efficiency of various distribution operations. Compliance with regulatory standards and the adoption of innovations such as EREA technology help to raise industry standards. In particular, this ensures that radiopharmaceutical products are distributed in accordance with the most stringent standards.
Would you like to find out more about our tool? Please feel free to leave us a message and contact our specialists at EREA.
Need a specific configuration? Customised dimensions?
Discover our insulators, tailor-made to meet your requirements.















